15K views View upvotes Related Answer Samiksha Singh. A chemical bond is a lasting attraction between atoms ions or molecules that enables the formation of chemical compounds.
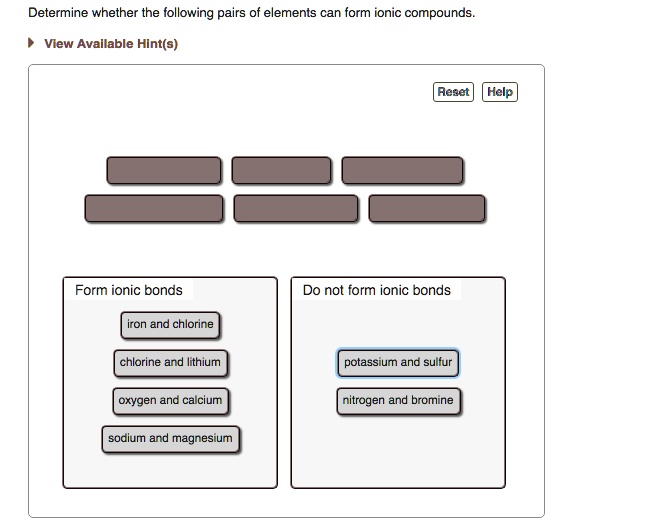
Solved Determine Whether The Following Pairs Of Elements Can Form Ionic Compounds Vlew Avallable Hint S Aeset Help Form Ionic Bonds Do Not Form Ionic Bonds Iron And Chlorine Chlorine And Iithium Potassium And
The ability of one specie willing to lose electron and the other gaining is the main bed rock of ionic bonding.
. Potassium will lose one of its electrons which will be gained by the Bromine. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds. A chemical bond is a lasting attraction between atoms ions or molecules that enables the formation of chemical compounds.
Ionizing it doesnt change Z aka the number of protons. A covalent bond forms when two non-metal atoms share a pair of electrons. Nitrogen bromide Br3N is a covalent bond What is chemical bond ionic bond covalent bond.
The electronegativity of K is 082 and that of bromine is 296 wick is a difference of 204 which also indicates that the bond would be ionic. Nitrogen has an Electronegativity of χ N3 Bromine of χ Br29. Bromine is a highly electronegative non-metal which is a halogen.
I would personally say non-polar as the electronegativity of both bromine and iodine are quite similar however the electrons would saturate bromine slightly more than iodine as the En electronegativity is slightly higher. An atom that shares one or. The ions do have one extra electron but the number of protons cant change without changing the atomic species.
The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. If the difference in electronegativity between two elements is greater than 17 the bond they form is largely ionic. 23K views View upvotes Related Answer Philip Howie materials scientist academic researcher.
Both a fluorine atom and a bromine atom gain one electron and both atoms become stable. And this is how you solve all of these homework problems. Chemical bond A chemical bond is a lasting attraction between atoms ions or molecules that enables the formation of chemical compounds.
Bromine needs to gain one electron to have noble gas configuration or 8 valence electrons. Thus bromine atoms and bromine ions all have 35 protons each. A force that holds atoms together is a chemical bond When sodium atoms Na and chlorine atoms Cl join to make sodium chloride or table salt they form an ionic bond.
Bromine Br2 is a Covalent bond. Using this information which pair of elements is most likely to form an ionic bond. The electrons involved are in the outer shells of the atoms.
The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds. H forms only one bond because it needs only two electrons. Or through the sharing of electrons as in covalent bonds.
What is chemical bond ionic bond covalent bond. Potassium needs to lose one so they make a perfect pair to transfer electrons in an ionic bond. The electrostatic attraction between the two species will cause the ionic bond to form.
Or through the sharing of electrons as in covalent bonds. A chemical bond is a lasting attraction between atoms ions or molecules that enables the formation of chemical compounds. Hydrogen is an exception to the octet rule.
The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds. A covalent bond is formed between two atoms by sharing electrons.

Solved Which Of The Following Pairs Of Elements Are Likely To Form Ionic Compounds Check All That Brainly Com
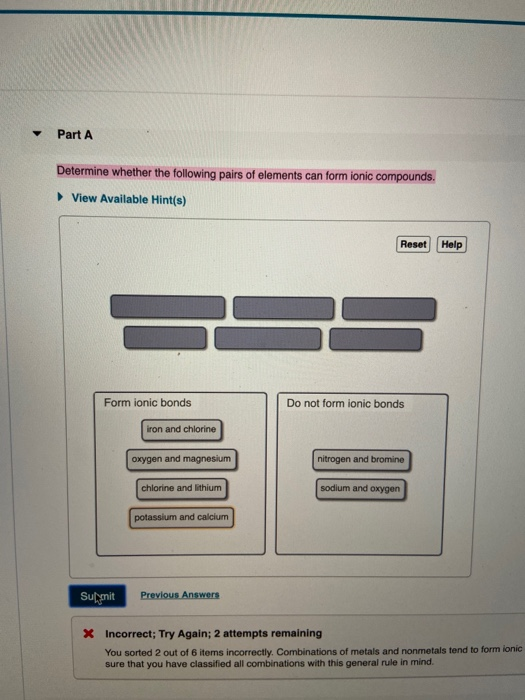
Solved Determine Whether The Following Pairs Of Elements Can Chegg Com

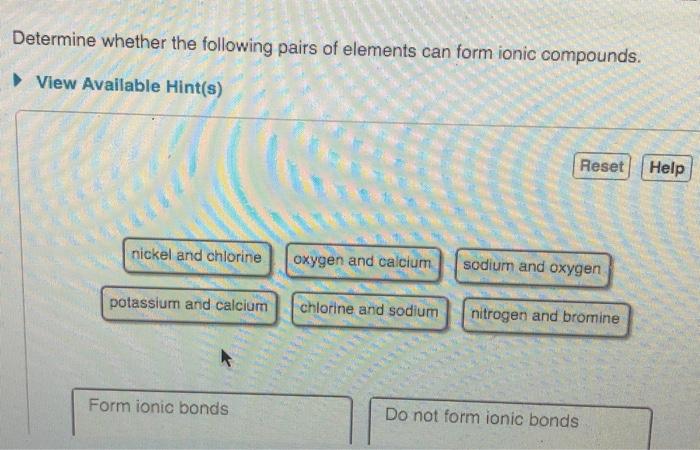
Solved Part A Determine Whether The Following Pairs Of Chegg Com
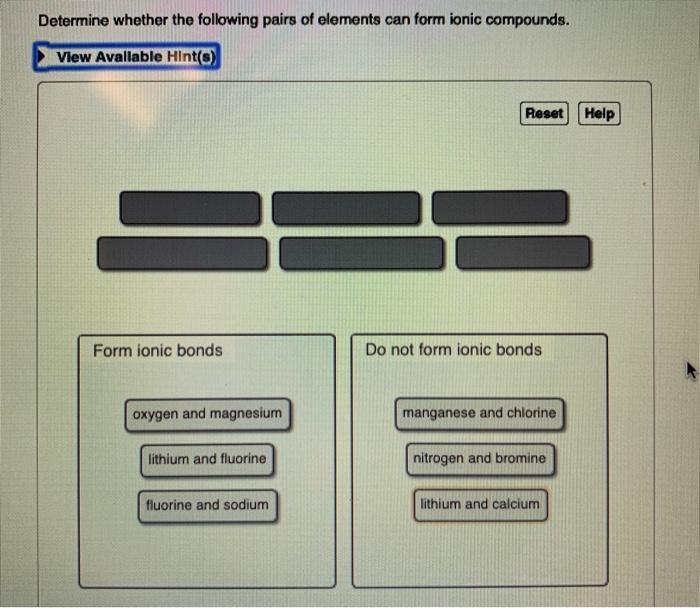
Solved I Thought This Is The Answer But It Was Wrong Would Chegg Com
0 Comments